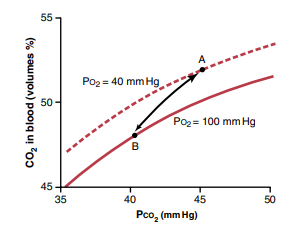
Describe Haldane's effect
Haldane’s effect is a phenomenon in which the oxygenation of hemoglobin in the blood affects its ability to carry carbon dioxide. Specifically, when hemoglobin is fully oxygenated, it has a reduced affinity for carbon dioxide, and as it releases oxygen to the tissues, its affinity for carbon dioxide increases.
Here are some key points to further describe the Haldane’s effect:
- Hemoglobin has two binding sites: one for oxygen and one for carbon dioxide. The binding of one molecule affects the binding of the other.
- When hemoglobin is oxygenated, it has a reduced affinity for carbon dioxide. This means that oxygenated blood can carry less carbon dioxide than deoxygenated blood.
- As hemoglobin releases oxygen to the tissues, it becomes more acidic due to the production of carbon dioxide through cellular respiration. This increase in acidity promotes the release of carbon dioxide from the tissues into the blood, where it can bind to hemoglobin and be transported to the lungs for exhalation.
- At the lungs, where oxygen levels are high, hemoglobin becomes more saturated with oxygen and its affinity for carbon dioxide decreases. This allows carbon dioxide to be released from hemoglobin and exhaled out of the body.
The Haldane’s effect is an important mechanism that helps regulate the levels of oxygen and carbon dioxide in the body. It ensures that oxygenated blood can efficiently transport carbon dioxide from the tissues to the lungs for exhalation, and that deoxygenated blood can efficiently transport oxygen from the lungs to the tissues for cellular respiration.
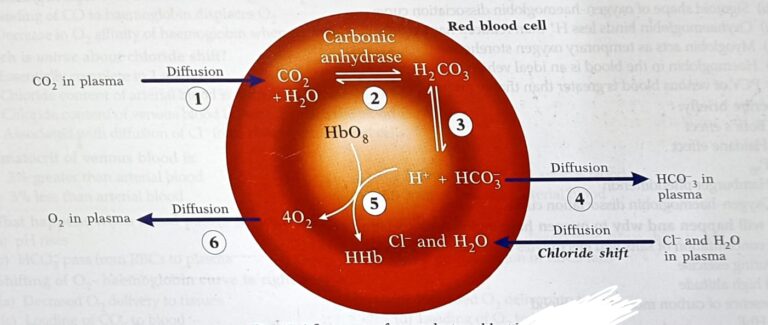
What is Chloride shift
CO2 is converted into bicarbonate inside the RBC and then diffuses into plasma -the steps involved are
CO2 in the tissues
↓
Enters into the blood
↓
Enters into the RBC
↓
Combines with H2O to form carbonic acid in the presence of enzyme “carbonic Anhydrase”
↓
Carbonic acid dissociates into bicarbonate ions (HCO3-) and Hydrogen ions (H+)
↓
Diffusion of bicarbonate into the plasma and H+ are buffered by hemoglobin Chloride Shift:
– Diffusion of HCO3- out of RBC into plasma
↓
less negative inside
↓
to neutralize this effect negatively charged chloride ions diffuse from plasma into the RBC
– This movement of chloride ions into the RBCs is called chloride shift
Significance of chloride shift
– maintains the membrane potential of RBC
– causes movement of other ions into RBC which is followed by osmosis of water
into RBC. This increases the volume of RBC in venous blood. This increases the
hematocrit value of venous blood.
Describe CO2 transport
Transport of CO2 in the blood
CO2 is transported in the blood in three forms
– In dissolved state (7%)
– In bicarbonate form (70%)
– In carbamino compound form (23%)
In dissolved form
0.3 ml of CO2 is transported from tissues to lungs in dissolved form
In bicarbonate form
CO2 is converted into bicarbonate inside the RBC and then diffuses into plasma -the steps involved are
CO2 in the tissues
↓
Enters into the blood
↓
Enters into the RBC
↓
Combines with H2O to form carbonic acid in the presence of enzyme “carbonic Anhydrase”
↓
Carbonic acid dissociates into bicarbonate ions (HCO3-) and Hydrogen ions (H+)
Diffusion of bicarbonate into the plasma and H+ are buffered by hemoglobin
Chloride Shift:
– Diffusion of HCO3- out of RBC into plasma
↓
less negative inside
↓
to neutralize this effect negatively charged chloride ions diffuse from plasma into the RBC
– This movement of chloride ions into the RBCs is called chloride shift
Significance of chloride shift
– maintains the membrane potential of RBC
– causes movement of other ions into RBC which is followed by osmosis of water into RBC. This increases the volume of RBC in venous blood. This increases the hematocrit value of venous blood.
In carbamino form
CO2 + Aminogroup of plasma proteins – Carbamino proteins
CO2+ Aminogroup of Hb – Carbaminoglobin
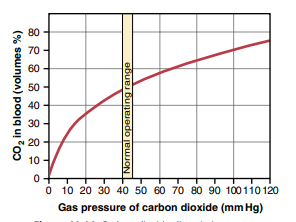
CO2 dissociation curve
– Curve obtained by plotting the relationship between PCO2 and total CO2 content of the blood
– the total CO2 content of the blood is directly proportional to PCO2 of the blood
Factors affecting the curve
1) Oxygen
– Increase in PO2 causes increase in CO2 dissociation (Haldane’s effect)
(loading of O2 in lungs causes unloading of CO2)
– Shifts the curve to right
2) 2, 3 – DPG
– Competes with CO2 to combine with Hb
– Shifts the curve to right
Delivery of CO2 in the lungs: Involves the following steps
1. Release of CO2 from carbaminohaemoglobin into plasma
Entry of O2 into RBC – Oxygenation of Hb
↓
Oxyhaemoglobin has low affinity for CO2
↓
Release of CO2
2. Bicarbonate is converted into CO2 by reverse chloride shift
Oxyhemoglobin releases H+ ions
↓
Entry of HCO3- from plasma into RBC in exchange for Cl- ions (Reverse chloride shift)
H+ combines with HCO3- to form carbonic acid
↓
Carbonic acid dissociates into H20 & CO2
↓
CO2 diffuses out of RBC into plasma
↓
Diffusion of CO2 from plasma to alveoli
– Difference in the partial pressure of CO2 between alveoli and pulmonary capillary blood makes the CO2 to diffuse out of blood into the alveoli
Amount of CO2 delivered in the lungs
CO2 content of venous blood – 52 ml/100 ml
CO2 content of Arterial blood – 48 ml/100 ml
So CO2 delivered into the lungs – 4 ml / 100 ml
Total amount of CO2 transported = 4 /100 X 5000 (cardiac output) = 200 ml/minute