What are the Hazards of blood transfusion
HAZARDS OF BLOOD TRANFUSION
Complications of whole blood transfusion
1. Hemolytic reaction due to red cell incompatibility
2. Transmission of certain diseases like hepatitis malaria, AIDS, Syphilis etc.,
3. Transient hyperkalemia followed by hypokalemia
4. Hypocalcemia
5. Volume overload
6. Bacterial contamination
7. Thrombophlebitis
8. Air embolismEffect of mismatched blood transfusion
Mismatching refers to transfusion of incompatible blood groups
a. Mild hemolytic reaction
Aggultination
↓
Hemolysis
↓
Bilirubin
↓
Jaundice
b. Severe immediate hemolytic reaction
Wide spread agglutination leads to development of following signs and symptoms.
1. Chill and rigors
2. Fever and headache
3. Breathlessness
4. Chest pain and abdominal pain
5. Nausea and vomiting
6. Joint pain
7. Circulatory shock (due to release of histamine and other vasodilators)
Feathers of post shock phase
8. Hemolysis of agglutinated cells
9. Release of hemoglobin and its excretion into the urine
10. Jaundice
11. Anemia
12. Renal shut down due to precipitation of Hb in the renal tubules
13. Hyperkalemia and uraemia
c. Delayed hemolytic reaction:
1. Occurs 3days to 3weeks after transfusion only as 1 in 3200 transfusions
2. Antibodies that develop against donor cells cause agglutination and hemolysis
3. Symptoms are often mild or absent.
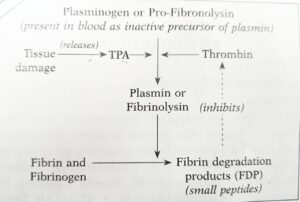
Add a note on fibrinolytic system in the blood
ANTICOAGULANT MECHANISM:
FIBRINOLYTIC SYSTEM
Factors that initiate clotting mechanism also stimulate the (break up) of the blood clot, called fibrinolysis.
Fibrinolysis is due to’ the action of proteolytic enzyme ‘fibrinolysin’ or ‘plasmin’. It is present in the circulation as inactive ‘plasminogen’ (profibrinolysin) which gets converted to “plasmin” by the action of ‘thrombin’ and tissue plasminogen activator – TPA (released by tissue damage). Plasmin lyses fibrin and fibrinogen, with the production of fibrinogen degradation products (FDP) that inhibit thrombin. However, this process of fibrinolysis is much slower than clotting process.
Factors Affecting Fibrinolytic System
(A) Promoted by
1. Stress and strains (physical or mental) e.g. violent exercise, surgical operation; that is why in violent sudden death, the blood is in fluid state and incoagulable as a result of fibrinolysis.
2. After administration of epinephrine, corticosteriods and phenformin (an anti-diabetic agent).
3. Tissue activators (occur
in microsomes)
are widely distributed throughout the body cells and body fluids e.g. urine contains a plasminogen activator, urokinase produced by kidney cells.
(B) Inhibited by ‘antiplasmin’ which prevents activation of plasminogen, e.g.
1. E-amino caproic acid (EACA)
2. Aprotinin or Trasylol (trypsin inhibitor).
Physiological Significance of
Fibrinolytic System
1. In physiological conditions the clotting system of plasma is continually forming small amounts of fibrin which is deposited to form a thin layer on vascular endothelium, and that the fibrinolytic system is constantly in action to prevent excessive fibrin formation, therefore,
(i) if clotting system predominates it leads to intravascular thrombosis; and
(ii) if fibrinolytic system predominates it leads to
tendency of bleeding.
The above (i) and (ii) normally remain balanced.
2. Plasmin can form ‘kinins’ (e.g. bradykinin, kallidin) which contribute to the vascular and sensory features (pain) of the inflammatory response to injury.
3. It also plays a role in cell movement and in ovulation producing defects in growth and fertility.
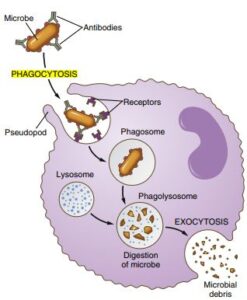
write about Physiology of phagocytic mechanism
PHYSIOLOGY OF
PHAGOCYTIC MECHANISM S/N
Neutrophils play an important role in inflammation, particularly bacterial. The following events are seen during phagocytosis.
1. Diapedesis. Neutrophils are motile cells, therefore, they leave from the blood stream into the tissues by passing through the junction between endothelial cells, a process called ‘diapedesis’
2. Chemotaxis. Bacterial products interact with plasma proteins to produce ‘agents’ that attract neutrophils to the site of injury or inflammation, called ‘chemotaxis’.
As a result of chemotaxis, neutrophils adhere to each other forming clumps and become immobile. The chemotactic agents include:
(i) components of complement system-C5a
(ii) some of leukotrienes and colony stimulating factors (originate from free fatty acids),
(iii) polypeptides from lymphocytes, basophils and mast cells.
(iv) toxins produced by microorganisms, and
(v) kinins produced by damaged tissues.
3. Opsonization. Opsonins are antibodies against bacteria. The principal opsonins are immunoglobulin of IgG group and complement proteins. They coat the bacteria and make them attractive to the phagocytes, a process called ‘opsonization’.
4. Phagocytosis
(i) Coated bacteria then bind to the receptors on the neutrophil cell membrane and get phagocytosed by a process of ‘endocytosis’, forming antigen antibody complex.
(ii) This way neutrophils also phagocytize many foreign substances e.g. carbon particles, sodium urate crystals, etc.
iii) Phagocytosis occurs at pH between 6 to 8 and is not dependent on O2. It is helped by bivalent cations and is prevented by calcium chelating agents.
(iv) A neutrophil can phagocytize 5-20 bacteria before the neutrophil itself become inactivated and dies.
(v) Macrophages are more powerful phagocytes than neutrophils. They can phagocytize as many as 100 bacteria; can engulf particles upto the size of a RBC; and have the ability to phagocytize necrotic tissues and even dead neutrophils.
5. Degranulation. After phagocytosing the bacteria , bacteriocidal substances (called defensins) such as lysozymes and peroxidases are released from the lysosomal granules into the digestive Pouch (degranulation), where they kill and digest the bacteria.
6. Inflammatory Response. Release of lysosomal enzymes, histamine and 5-HT into CF produces inflammatory response.
7. Limiting Inflammation. Fusion of phagocytic vacuole with lysosome causes release of:
(i) ‘Thromboxanes’ produces
(a) vasconstriction and
(b) platelet aggregation (stick to each other)
(ii) ‘Prostaglandins’ produces: anti-inflammatory
effect.
(i) and (ii) limit the inflammation.
write short note on PURPURA
1. Clotting Time (CT): Normal (3-8 minutes).
2. Bleeding Time (BT): Increases (normal: 2-5 minutes).
3. Capillary endothelium resistance: decreases, causing increased capillary fragility.
Evidense: If firm pressure is applied to the skin (by inflating a blood pressure cuff at 60 mmHg for 2 minutes) or a suction force (by the negative pressure used in ‘cupping’) the local capillaries leak blood, leading to appearance of a crop of minute haemorrhage (petechiae).
4. Skin Microscopy
(i) In primary purpura – skin capillaries are very irregular and distorted in form, sometimes branching; after puncture these vessels remain patent, therefore, free bleeding proceeds from the needle track for several minutes.
(ii) In secondary purpura the capillaries are anatomically normal but because of presence of toxic agents or other causes they do not contract effectively in response to injury.
5. Platelet Count: (Normal 1.5-4.0 lacs/ul In many cases of purpura there is reduction in platelet- count, called thrombocytopenic purpura. Also occurs in hypersplenism because platelets are destroyed in spleen.
6. With low platelet count, clot retraction is deficient and there is poor constriction of ruptured vessels. The clot formed is soft, friable and does not retract well. This results in easy bruisability and multiple subcutaneous haemorrhages.
#Forms/Classification
1. Thrombocytopenic Purpura: Purpura with low platelet count. It results in poor clot retraction and poor constriction of injured blood vessels, therefore, it is characterized by easy bruisability and subcutaneous haemorrhages. Clinically, it is seen as:
(i) Mild purpura: platelet count less than 50,000/μL.
(ii) Moderate purpura: platelet count less than 10,000/μL. It is characterized by ‘severe bleeding’.
(iii) Fulminating purpura: platelet count less than 1000/μL.
2. Athrombocytopenic Purpura: purpura with normal platelet count.
3. Thromboasthenic Purpura: It is due to abnormal circulating platelets but platelet count is normal.
4. Haemorrhagic Telangiectasis: It is entirely due to a localized capillary abnormality. There is group of dilated capillaries in the skin or mucous membrane; these do not contract with stimuli which affect normal capillaries. Profuse bleeding follow rupture of these vessels.
What is Landsteiner's law
A and B are group specific substances, polysaccharide in nature. They are called antigen (agglutinogen) i.e. in the presence of a suitable antibody (agglutinin or ‘isohaemagglutinin) cause clumping of RBCs (agglutination).
The agglutinin acting on agglutinogen A is called ‘a’ or ‘Anti-A’; the agglutinin acting on agglutinogen B is called ‘B’ or ‘Anti-B’. Group specific substance ‘O’ does not normally act as an agglutinogen and there is no corresponding agglutinin; that is why group ‘O’ RBCs are not agglutinated by agglutinins a or B. The agglutinins a and B are globulins of IgM type and cannot cross the placenta.
Based on these facts Karl Landsteiner in 1900 framed a law, called Landsteiner’s law. It has two major components:
1. If an agglutinogen is present in the RBCs of an individual, the corresponding agglutinin must be absent from the plasma;
2. If the agglutinogen is absent in the individual RBCs, the corresponding agglutinin must be present in the plasma.
Exception to the 2nd part are: absence of Rh agglutinogens from the RBCs which are not accompanied by presence in the plasma of anti-Rh, agglutinins.
Write short note on Rh incompatibility
Rh incompatibility occurs when the blood of a mother who is Rh-negative (lacks the Rh factor protein on her red blood cells) comes into contact with the blood of her Rh-positive fetus. This can cause the mother’s immune system to produce antibodies against the Rh factor, which can cross the placenta and attack the fetus’s red blood cells, leading to a condition called hemolytic disease of the fetus and newborn (HDFN).
In mild cases, the fetus may have mild anemia, while in severe cases, it can lead to hydrops fetalis, a condition in which the fetus accumulates fluid and may die before or shortly after birth. Fortunately, Rh incompatibility can be prevented with Rh immune globulin injections, which prevent the mother’s immune system from developing antibodies against the Rh factor. If HDFN is detected, treatment may include in utero blood transfusions, early delivery, or exchange transfusions after birth.
Rh incompatibility is a rare but serious condition that can be easily prevented with appropriate medical care, making it important for pregnant women to receive regular prenatal care and testing for blood type and Rh status.
what Reactions due to incompatible blood transfusion?
Acute hemolytic transfusion reaction: This occurs when the recipient’s immune system recognizes the transfused red blood cells as foreign and attacks them, leading to the destruction of red blood cells, the release of hemoglobin, and the formation of blood clots that can block blood vessels and cause kidney failure. Symptoms include fever, chills, back pain, nausea, vomiting, and hypotension.
Febrile non-hemolytic transfusion reaction: This occurs when the recipient’s immune system reacts to white blood cells or platelets in the transfused blood, causing fever, chills, and mild hypotension.
Allergic reaction: This occurs when the recipient’s immune system reacts to proteins in the transfused blood, causing hives, itching, and anaphylaxis in rare cases.
Transfusion-associated circulatory overload: This occurs when the volume of transfused blood exceeds the recipient’s circulatory capacity, leading to fluid overload, pulmonary edema, and cardiac arrest in severe cases.
- Transfusion-transmitted infections: This occurs when the transfused blood contains viruses, bacteria, or other pathogens, leading to hepatitis B and C, HIV, and other infectious diseases.

